(G S Manku, email: manku,gs@gmail.com)
The branch of science dealing with the extraction of metals from their naturally occurring ores is called metallurgy.
17.1.1 ORIGIN OF UNIVERSE
Current popular theory for the origin and evolution of Universe starts with hot big bang. All matter in Universe was once present in a primeval nucleus of tremendous density and temperature, and for some unknown reason, exploded and distributed matter uniformly throughout space. The initial density of Universe was about 1093 kg m–3 and temperature was about 1032 K. After 1 second, the temperature fell to about 1010 K and elementary particles (protons, neutrons and electrons) were formed. For the next 10 to 500 seconds after the big bang, conditions similar to those in nuclear fusion reaction prevailed throughout the Universe, and elementary particles started combining to form deuterium and helium nuclei. Soon about 25 % of the mass of Universe was converted to helium and about 0.001 % deuterium. From this material, the earliest stars were formed and synthesis of other elements began. Assuming that the rate of expansion of Universe as 18 km–1 per light year, it is estimated that the big bang occurred about 1.8 × 1010 years ago.
Fortunately, for our purposes, we distinguish between the origin of matter (cosmology) and origin of elements (nucleogenesis). The isotopic distribution within the Universe, the solar system and on Earth is independent of cosmology and can be interpreted on the basis of observations and principles of physics. Information about abundance of elements in sun and stars, gaseous nebulae in this and other galaxies, and interstellar medium can be obtained by spectroscopy. A direct analysis of elements is possible in cosmic rays, meteorites and surface regions of Earth. Though there are differences in relative abundance of different elements in different places, overall, about 99 % of all elements present in Universe is hydronium (hydrogen).
Solar system is dominated by Sun. Analysis of atomic absorption lines from solar spectra indicate that in Sun, hydrogen (88.6 %) and helium (11.3 %) are the most abundant elements, followed by less than 0.1 % oxygen and carbon.
17.1.2 ABUNDANCE OF ELEMENTS
Most elements are obtained from the minerals present in Earth’s crust. Earth’s crust is comparatively very thin in comparison with the Earth’s central core and mantle. In earth’s crust, though some elements are present in abundance, e.g., oxygen (46 % by mass) and silicon (27 % by mass), others are present in amounts less than even 1 part in 1010 parts. Eight most abundant elements in the Earth’s crust are oxygen (46.6 %), silicon (27.7 %), aluminum (8.1%), iron (5.0%), calcium (3.6%), sodium (2.8%), potassium (2.6%). and magnesium (2.1%), which make up about 98.5 % of total mass of Earth’s crust.
Some elements (promethium, technetium, rhenium, astatine, francium, etc.) do not occur in nature in any mineral, and have been prepared synthetically in Laboratories.
The most abundant element in human body by mass is oxygen (65 %), followed by carbon (18 %) and hydrogen (10 %).
Many attempts have been made from time to time to estimate the relative abundance of the elements in Universe, notably by Goldschmidt (1931), Brown (1949) and Urey (1952). A plot of log A (A = cosmic abundance of the element) against atomic number Z of the elements (Figure 17.1.1) shows that:
X =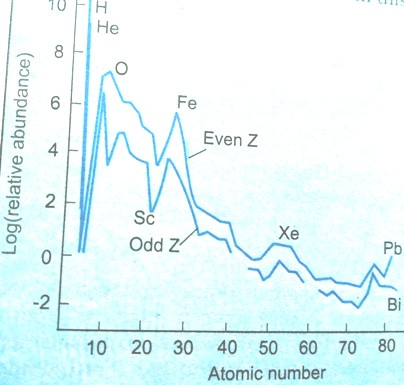
Figure 17.1.1 Abundance of elements in nature plotted
against atomic number Z
- Elements with even atomic numbers are more abundant than their neighboring elements having odd atomic numbers.
- Elements having mass numbers divisible by 4 are more abundant 4He, 12C, 16O, 20Ne, 24Mg, 28SI, 32S and 40Ca (Rule of Oddo, 1914).
- The log A falls rapidly and almost regularly up to Z = 48, after which the variation in abundance is smaller.
- Deuterium, lithium (Z = 3), beryllium (Z = 4) and boron (Z = 5) have very low abundance as compared to the neighboring elements.
- Abundance of iron (Z = 26) is very high.
- Abundance of elements round Z = 26 (iron), 56 (barium) and 78 (platinum) is more than that expected from the general trends in the curve.
- Atoms of heavier elements are neutron rich.
17.1.3 OCCURRENCE OF METALS
Most elements are obtained from the minerals present in Earth’s crust. Earth’s crust is comparatively very thin in comparison with the Earth’s central core and mantle. In earth’s crust, though some elements are present in abundance, e.g., oxygen (46 % by mass) and silicon (27 % by mass), others are present in amounts less than even 1 part in 1010 parts. Some elements (promethium, technetium, rhenium, astatine, francium, etc.) do not occur in nature in any mineral, and have been prepared synthetically in Laboratories.
Except for very unreactive or noble metals like silver, gold, and the platinum metals, all other metals occur in nature in form of their stable compounds. The compounds are always mixed with undesirable siliceous matter called gangue. A mineral of the element is defined as a mixture of the naturally occurring compound with the gangue. It is generally a hard crystalline solid with definite crystal shape and has almost a fixed composition, so that it can be assigned a chemical formula. An ore of the metal is the mineral, which is suitable for the commercial extraction of the element. Thus, all the ores are minerals, but all minerals are not ores. For example, even though calcium, aluminium or magnesium occurs plentifully in nature as the silicate rocks, the latter are not ores for either of these metals.
On the other hand, there are many minerals, which have their own commercial value are used as such. Examples include gypsum, CaSO4.2H2O, calcite or marble, CaCO3, and beryl 2BeO.Al2O3.6SiO2, which is a precious stone and has gem value.
17.1.3.1 Classification of Metals
On the basis of the chemical nature of compound which exists in nature, Hulme has classified the metals into the following categories.
- Type I Metals: These are the very electropositive elements (the alkali metals (the group 1, ns1 elements) and beryllium (group 2, the 2s2 element). They have a very high negative standard reduction potentials (E0 = –2.5 V to –3 V), and exist in nature as soluble but hard crystalline chlorides, sulphates and carbonates.
- Type II Metals: These are alkaline earth metals (group 2, ns2 elements) and occur mainly as insoluble sulphates and carbonates. These elements are also very electropositive and have a high negative E0 values (–2.37 V to –2.95 V). Magnesium with electronic configuration of ns2, occurs in nature as soluble chlorides (carnallite MgCl2.6H2O) and sulphate (epsomite MgSO4.7H2O) as well as insoluble carbonates (magnesite MgCO3 and dolomite CaCO3.MgCO3).
- Type III Metals: These are group 3, 4, 5 and 13 elements having d1, d2, d3 and ns2np1 configuration, chromium (3d54s1) and manganese (3d54s2). These are also electropositive, but much less reactive than the group 1 and group 2 elements. Their E0 values are negative, which are less in magnitude than for alkali or alkaline earth metals. These elements do not have any lone pair of electrons in their d orbitals that are used for the formation of a d–p–π bond. Hence, they exist mainly as oxides and mixed oxides.
- Type IV Metals: These metals have the electronic configuration of (n – 1)d6-10 ns2. They have lone pairs of electrons in their d orbitals, and form a stable d–p–π bond with sulphur atoms. Hence, these metals occur as stable sulphides, and less frequently, as oxides, for example, molybdenum, iron, cobalt, nickel, copper, silver and post-transition p block metals (arsenic, antimony, bismuth, lead etc.
- Type V Metals: Due to low reactivity (reduction potentials are positive (Eo > 0.8$ V) and instability of oxides and sulphides, these elements do not form any stable compound in nature and occur in nature as free elements. Examples include gold, silver and platinum metals.
The classification is not rigid and many borderline cases exist. For example,
- Iron ( 3d64s2 element) occurs as oxides (hematite, Fe2O3 or magnetite, Fe3O4), carbonate (franklinite FeCO3) as well as sulphides (pyrites FeCuS2 ), but is extracted mainly from its oxide ore, hematite;
- Copper (3d104s1 element) occurs as oxide (cuprite Cu2O), carbonates (malachite, CuCO3.Cu(OH)2 or azurite CuCO3.2Cu(OH)2) as well as sulphides (pyrites FeCuS2, or copper glance CuS2).
- Zinc (3d104s2 element) occurs as sulphide (zinc blende, wurtzite or sphalerite ZnS), oxide (zincite ZnO) as well as carbonate (calamine ZnCO3).
- Lead (5d106s26p2 element) occurs as carbonate , oxide (litharge PbO) as well as sulphide (galena, PbS).
- Silver (4d105s1 element) occurs as chloride (horn silver AgCl) as well as sulphide (argentite AgS2).
- To some extent, mercury, silver and copper occur in native state also.
Table 17.1.1 Important Ores and Methods of Extraction of the Metals
| Electrode
Potential (V) |
Element |
Important Ore of the Metal |
Method used for Extraction |
| – 3.04 V | Li | Spodumene LiAl(SiO3)2 | Electrolysis of fused LiCl |
| – 2.92 V | K | Carnallite KCl.MgCl2.6H2O Indian Saltpeter KNO3 |
Electrolysis of fused KOH |
| – 2.90 V | Ba | Barytes BaSO4 Witherite BaCO3 |
Electrolysis of fused BaCl2
|
| – 2.89 V | Sr | Strontianite SrCO3 Celestine SrSO4 |
Electrolysis of fused SrCl2
|
| – 2.87 V | Ca | Limestone CaCO3 Gypsum CaSO4.2H2O |
Electrolysis of fused CaCl2 in CaF2 |
| – 2.7 V | Na | Rock salt NaCl Chile saltpeter NaNO3 |
Electrolysis of fused NaCl in CaCl2 |
| – 2.37 V | Mg | Carnallite KCl.MgCl2.6H2O Magnesite MgCO3 Dolomite CaCO3.MgCO3 |
Electrolysis of fused MgCl2 in KCl or reduction of MgO with coke ( Reversible) |
| – 1.7 V | Be | Beryl 2BeO.Al2O3.6SiO2 | Electrolysis of fused BeF2 in KF or reduction of fluoride with potassium |
| -1.66 V | Al | Bauxite Al2O3.2H2O | Electrolysis of fused oxide in Na3AlF6 (cryolite) |
| – 1.2 V | Mn | Pyrolussite MnO2 Hausmanite Mn3O4 |
Reduction of oxide with Al or coke |
| – 0.95 | Ti | Rutile TiO2
Ilmenite FeTiO3 |
Reduction of chloride with
Mg or Na |
| – 0.76 | Zn | Zinc Blende ZnS Zincite ZnO, Calamine ZnCO3 |
Reduction of ZnO with coke or, electrolysis of aqueous ZnSO4 solutions |
| – 0.74 V | Cr | Chromite Fe(CrO2)2 | Reduction of oxide with Al |
| – 0.44 V | Fe | Hematite Fe2O3 Siderite FeCO3 |
Reduction of oxide with coke
|
| – 0.28 V | Co | Smaltite CoAsS2 | Reduction of oxide with coke or aluminium |
| – 0.25 V | Ni | Milerite NiS | Reduction of oxide with C, CO or hydrogen |
| – 0.14 V | Sn | Cassiterite SnO2 | Reduction of SnO2 with coke |
| – 0.13 V | Pb | Galena PbS | Reduction of PbO with coke |
| + 0.32 V | Bi | Bismuth glance Bi2S3 Bismuthite Bi2O3 |
Reduction of Bi2O3 with coke |
| + 0.34 V | Cu | Pyrites CuFeS2 Cuprite Cu2O Malachite CuCO3.Cu(OH)2 |
Autoreduction of Cu2S with
Cu2O or electrolysis of aqueous CuSO4. |
| + 0.80 V | Ag | Argentite Ag2S Horn silver AgCl |
Cyanide extraction and electrolysis of cyanide solution |
| + 0. 85 V | Hg | Cinnabar HgS | Thermal decomposition of oxide |
| + 1.20 V | Pt | Native platinum Pt Sperylite PtAs2 |
Thermal decomposition of (NH4)2PtCl6 |
| +1.50 V | Au | Native gold Au Sylvelite Au2S |
Cyanide process like that for Silver |
Note: The table does not represent all the elements. Also, it does not represent all the ores or minerals for an element. The table is only representative of main procedure adopted for the extraction of the element.