(G S. Manku, email: manku.gs@gmail.com)
(4.1).1 EARLY CLASSIFICATION OF ELEMENTS
After Boyle’s theory of atoms (1677) as small sized particles of different shapes, and Dalton’s laws of chemical combination (1808), attempts were made by scientists to arrange or form groups of known elements having similar properties.
- In 1829, J W D, J W Döbereiner pointed out triads of elements (a group of three elements) having similar properties, in which the mass of one element was average mass of the other two members. Some triads known at that time were, lithium (7), sodium (23) and potassium (39); calcium (40), strontium (88) and barium (137); chlorine (35.5), bromine (80) and iodine (127); sulphur (32), selenium (78) and tellurium (126).
- In 1850, Pattenkofer showed that masses of similar elements differed by almost a multiple of eight. For example, lithium (7), sodium (23) and potassium (39); calcium (40), strontium (88) and barium (137); boron (9) and aluminium (27), carbon (12) and silicon (28); oxygen (16), sulphur (32), selenium (78) and tellurium (126).
- In 1862, Chancourtois modified Pattenkofer’s idea by changing the difference in masses to be a multiple of 16.
- In 1864, J A R Newlands proposed his law of octaves of elements: When elements are arranged in the order of increasing atomic masses (or atomic weights), every eighth element has similar properties. For satisfying the law proposed, he left vacant spaces for the ‘undiscovered elements’; and was ridiculed for this by the scientific community.
(4.1).2 Mendeleev’s Periodic Table of Elements
(4.1).2.1 The Mendeleev’s Table
In 1868–1870, Demitri Mendeleev published a series of tables of elements, in which the elements were arranged according to their increasing atomic weights (or, atomic masses. A modern version of the Mendeleev’s periodic table is given as Table 4.1.1. It has the following salient features.
Groups: The elements are arranged in nine vertical columns, called groups, which were numbered as I, II, III, …, VIII, and 0 (zero). Except for group VIII and group zero, each group was subdivided into two sub-groups, A and B. The elements of a particular subgroup show similar properties with a regular gradation from top to bottom.
Periods: The horizontal rows of the table are called periods. In a period, the properties of the elements show a regular change. (See below for details.)
Place for unknown elements: To assign a suitable groups to an element, in which all the members have similar properties, vacant spaces were left to accommodate the yet undiscovered elements.
Change of atomic masses (atomic weights): In some cases, to fix it in a suitable place, the valency of the element was changed arbitrarily without any fresh chemical evidence. The following example will make this clear.
In 1870, aluminium was considered divalent metal and its oxide was assigned the formula AlO. The Al:O mass ration in the oxide is 9:8 (54:48). Therefore, the equivalent weight of aluminium was taken as 9, being the mass of aluminium, in grams, combining with 8 grams of oxygen. Therefore, accepted atomic weight of aluminium was considered as 2 × 9 = 18, which was between the atomic masses of two highly electronegative elements, oxygen (atomic mass = 16) and fluorine (atomic mass = 19). To assign a better place for aluminium, Mendeleev changed its valency to 3, which made its atomic mass = 3 × 9 = 27, and placed it conveniently between electropositive magnesium (24) and silicon (28) in the periodic table.
However, many elements like cerium, thorium or uranium remained placed incorrectly in the table because of (1) erroneous results accepted and (2) between hydrogen and uranium, as many as 26 elements were yet undiscovered at that time.
Table (4.1).1 A Modern Version of Mendeleev’s Table of Elements

* The 14 elements following lanthanum (La), called lanthanons or the lanthanide elements, are grouped together and placed along with lanthanum in sixth period of group III B.
** The 14 elements following actinium (Ac), called actinons or the actinide elements, are grouped together along with actinium, and placed in the seventh period of group III B.
(4.1).2.2 Advantages of Mendeleev’s Periodic Table
The Mendeleev’s table of elements proved helpful in understanding the variations and gradation in the properties of different elements and their compounds.
1.Prediction of Properties: From the gaps present in the table, Mendeleev predicted properties of three undiscovered elements, heavier than but similar to boron, aluminium, and silicon. When scandium (Sc), gallium (Ga) and germanium (Ge) were discovered, their properties were remarkably predicted by him. This made Mendeleev’s periodic table became extremely popular for his remarkable insight and accuracy.
2. The group number of the element indicated the highest valence state attained by the element.
3. Gradation in Properties: In a subgroup, the elements and their compounds show a regular gradation in their physical and chemical properties (See later for details).
4. Diagonal Relationship: There are three pairs of elements belonging to different groups, which resemble each other very closely: (1) lithium (Li) – magnesium (Mg), (2) beryllium (Be) – aluminium (Al) and (3) boron (B) – silicon (Si). Each of these pairs has one element of group I, II and III of the second period and another element of third period placed diagonally to it in groups II, III and IV respectively. This change from highly electronegative halogens of group VII A to highly electropositive alkali metals of group I A indicated the existence of a group of elements, which should be neither electronegative nor electropositive. Discovery of noble gases in 1886, which had atomic masses between those of the halogens and alkali metals, gave considerable support to the Mendeleev’s table.
(4.1).2.3 Drawbacks of Mendeleev’s Periodic Table of Elements
The Mendeleev’s table has certain drawbacks, some of which are given below.
1. Length of a Period: There is no explanation for different number of elements in different periods. The extra-short first period has only two elements, short second and third periods have eight elements each, long fourth and fifth periods have eighteen elements each, and extra-long sixth and seventh periods} have 32 elements each.
2. Elements of A and B subgroups: Except for the formal similarity in the composition of the compounds of elements of A and B subgroups, there is almost no resemblance in their nature or In groups IV, V, VI and VII, the subgroup A contains electronegative elements, whereas the subgroup B contains metallic elements.
3. Group VIII has three subgroups, A, B and C, whereas group zero has only one subgroup. All other groups have two subgroups each. Why?
4. Only in group VIII, the members of subgroups A, B and C have three successive elements of the series. In all other cases, the successive elements are placed in two adjacent groups.
5. Stability of Different Valence States: It cannot be explained why for some elements, the higher states are stable, whereas for others, the lower states are more stable. Also, why should the valence state of elements of A subgroup changes by 2 units, whereas that for B subgroup elements changes by one unit?
6. Oxidation States: Though the group number indicates the highest oxidation state attained by the elements, in group VIII, only ruthenium and osmium show the octavalent state in teteroxide.
7. Group zero elements show oxidation states of II, IV, VI and VIII in fluorides, oxides and oxido anions, but no compound is formed in oxidation state of zero.
8. Zerovalent State: It is difficult to understand the concept of zero state in compounds, which exists in many metal carbonyls, nitrosyls and related organometallic compounds
9. Isotopes and Isobars: Isotopes of an element with different masses are clubbed together in one place, while isobars (same atomic masses, different elements) are assigned separate groups.
10. Reversal of Order of Elements: Simply for assigning proper groups to the elements, Mendeleev placed tellurium (atomic mass = 128) before iodine (atomic mass = 127); and cobalt (atomic mass = 59) before nickel (atomic mass = 58), without assigning any other reason.
11. Nature of the elements changes suddenly from highly electronegative halogens to highly electropositive alkali metals as atomic masses increase.
12. The Lanthanons and Actinons: In the sixth period, lanthanum (La, Z = 57) and the 14 elements following lanthanum with = 58 to 71), called lanthanons or lanthanides, are bunched together in one place in group B. Similarly, in seventh period, actinium (Ac, Z = 89) and the following 14 elements with Z = 90 to 103, called actinons or the actinides are bundled together and placed collectively in group III B.
13. Irregular changes in atomic masses: Finally, there is no regularity in the change in the atomic masses of the successive elements which may decrease by 1 unit (nickel and iodine) or increase by 1, 2, 3, 4 (argon, krypton, admium or xenon) or even 5 units (scandium, copper) for the successive elements. Therefore, there is no way of determining the number of elements between two known elements. The problem became serious in the studies of lanthanide elements – much effort was wasted in futile work in the field.
This shows that the classification of the elements on the basis of their atomic masses is not correct.
(4.1).3 PERIODIC LAW
(4.1).3.1 The Law
Moseley’s work on the characteristic X rays of elements showed that the atomic number Z, the number of protons in the nucleus of an atom, is a fundamental characteristic property of the elements. The number of electrons in the extranuclear part of an atom is also equal to Z. As properties of the elements and their compounds depend upon the arrangement of electrons in their extranuclear part, the properties of the elements strongly depend on their atomic number, Z.
Also, for all the successive elements, Z increases exactly by one unit.
According to the periodic law, the properties of elements are a periodic function of their atomic numbers.
(4.1).3.2 Bohr – Bury’s Long Form of Peroidic Table
(4.1).3.2.1 The Table
In the long form of the periodic table (Table 4.2.1), also known as the Bohr–Bury periodic table, elements are arranged in the order of increasing atomic numbers. The elements are arranged in such a manner that the configuration of their incompletely filled (valence) orbitals is the same.
Table (4.1).2 The Bohr – Bury’s Long Form of the Periodic Table
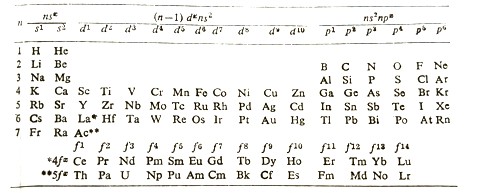
Note: The d orbitals filled are of the (n = 1)th shell, whereas the f orbitals (4f and 5f series) belong to the (n = 2)th shell and are the members of sixth and seventh groups respectively.
The salient points of the Long form of Periodic table are the following.
- The table consists of vertical groups and horizontal periods
- A new n-th period begins when the n-th shell, having principal quantum number = n, gets its first electron. From the auf bau principle for the electronic configuration of atoms, a period begins when the electronic configuration of the outermost occupied shell is n1.
- A period ends just one element before the outermost configuration becomes ns1. Therefore, the n-th period terminates when the electronic configuration of outermost shell of the atom becomes ns2np6. However, the first period (n = 1) ends at 1s2, because there are no p orbitals in the st shell of electrons.
This explains the different number of elements in a period as shown in Table (4.1).3.
Table (4.1).3 Number of Elements in different Periods of the Long Table
—————————————————————————————
Period Orbitals filled No, of Orbitals Total Electrons filled
—————————————————————————————-
First 1s 1 2
Second 2s, 2p 1 + 3 = 4 8
Third 3s, 3p 1 + 3 = 4 8
Fourth 4s, 3d, 4p 1 + 5 + 3 = 9 18
Fifth 5s, 4d, 5p 1 + 5 + 3 = 9 18
Sixth 6s, 4f, 5d, 6p 1 + 7 + 5 + 3 = 16 32
Seventh 7s, 5f, 6d, 7p 1 + 7 + 5 + 3 = 16 32
————————————————————————————-
(4.1).3.3 Advantages of The Long Form of Periodic Table
The advantages of the long periodic table over Mendeleev’s table are the following.
- The elements are arranged in order of increasing atomic numbers Z in such a way that in a group, all the elements present have similar electronic configurations of the incompletely filled outermost shells.
- All the members in a group have same electronic configuration of the outermost incompletely filled shells. Therefore, in the long periodic table, the elements of subgroups A, B as well as C of Mendleev’s table are assigned separate groups.
- The lanthanons (4f elements) and the actinons (5f elements) are not clubbed together, but are placed separately at the bottom of the table. Every element is given a separate place in the nf series of elements.
- As the properties of elements depend upon their electronic configurations, which is the basis of long form of table, the table is in a better position to explain the stability and existence of different oxidation states. It can explain the uniform divalent state for the 3d transition elements and the uniform trivalent state for the f block of elements.
- Noble Gases: The noble gases (configuration ns2np6) have a closed-shell octet of four electron pairs. They are noble because of (1) non-availability of low energy electrons for ionization or sharing with other atoms, and (2) absence of low energy vacant orbital for accepting electrons from other atoms.
Tendency of atoms to attain the stable noble gas shell by gain or loss of electrons in the valence shells makes the elements electronegative or electropositive.
(4.1).3.4 Limitation of the Long Form of Periodic Table
The long form of the periodic table is based upon the auf bau principle. Therefore, it incorporates all the limitations of the auf bau principle. Some of the limitations of the cumbersome and long periodic table are the following.
- Transition Elements: Electronic configuration of all the elements in a group may not be same. The elements of 3d and 5d series have two electrons in the outermost 4s or 6s shell (exceptions are chromium and copper in 3d series, and iridium, platinum and gold in 5d series). However, for the 4d elements, except for yttrium and zirconium, the outermost 5s shell contains only one electron. (See the electronic configuration of elements.)
- Lanthanide and Actinides – the 4f and 5f series: These elements are thrown out of the main body of the table as if they do not behave as expected from their configurations. It is impossible to determine their electronic configurations unambiguously and these elements may not have the expected configuration of (n – 2 )f1-14(n – 1 )d1 ns2.
- Though (1) lanthanum (La, Z = 57) and actinium (Ac, Z = 89) do not have any d electron, lanthanum and actinium are placed as the first elements of the 4d and 5d series) they resemble the f block elements, and (2) the 4f and 5f elements show a strong resemblance to these elements.
- Isotopes of Hydrogen: The isotopes of hydrogen have such a large difference in their properties that they are given different names, e.g., deuterium (symbol: D) for isotope with atomic mass = 2, and tritium (symbol: T) for isotope with atomic mass = 3. Yet, these isotopes are clubbed together in the periodic table like other isotopes.
- Half-filled and Completely-filled Subshell Configurations: There are elements (Cr, Mo, W, Cu, Ag, Au, Pd, Ir and Pt) for which the stability of half-filled and completely-filled subshell gives a configuration different from that predicted by auf bau However, this stability of configurations is not reflected in the chemical properties of the elements, except, perhaps for silver. (The stability of these configurations is explained on the basis of the Exchange energy as well as electrostatic considerations.)
- Borderline Elements: The borderline elements between s and d (scandium, yttrium, lanthanum and actinium) and between d and p (zinc, cadmium and mercury) have properties which are simple extrapolations of properties of the adjacent elements on either side. They are actually bridging elements between the two different types of elements, and but are classified as d elements.