(Author: Dr G S Manku, email: manku.gs@gmail.com)
It is difficult to determine and quantify the tendency of molecules to donate electron pairs to form a coordinate bond. Various methods like gas phase ionization or dissociation, calorimetric heats of solutions, displacement reactions,have been used for the purpose. However, some observed trends can be generalized.
1.6.1.1 Position in Periodic Table
Strength of Lewis base decreases with increasing electronegativity of the donor atom:
Me3N > Me2O > MeF
(Me3N readily displaces Me2O from the adduct Me2O:BF3).
For Lewis acids, trends are not simple, but for nitrogen and oxygen donor bases, the trend parallel the tendency for hydrolysis:
B > Be > Li ; Be > Mg > Ba, etc.
1.6.1.2 Effect of Substituents
The inductive, delocalization of electrons (resonance or mesomeric effects) and steric effects can be used for predicting the trends in the electron donation and electron acception. An electron withdrawing group will increase the strength of Lewis acids and decrease the strength of Lewis bases. Electron releasing groups will have just the reverse effect.
Me3N > Me2NH > MeNH2 > NH3 > NCl3 > NF3
(Base strength)
BF3 > B2H6 > BMe3
(Acid strength)
1.6.1.3 Delocalization of Electrons (Resonance or Mesomeric Effects)
The observed order of acid strengths BF3 < BCl3 < BBr3 or (MeO)3B < BMe3 is not the order expected due to inductive effects. This is due to the resonance stabilization of BF3 or B(OMe)3 as compared to BCl3 or BMe3:
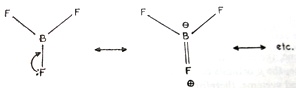
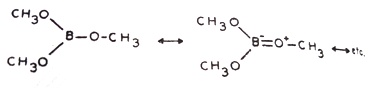
FIGURE 1.6.1 Canonical forms of boron trifluoride
and trimethoxyborane
Hyperconjugation effect is similar to the resonance effect and changes the acid and base strengths of Lewis acids and bases. For example, BF3 is normally stronger acid than diborane (H3B–BH3), and does nor form any complex with carbon monoxide, CO. But, diborane combines with CO to form an adduct which is stabilized by hyperconguation (Figure 1.6.2).

Figure 1.6.2 Hyperconjugation in H3B.CO and BH3..SMe2 adduct
Similarly, dimethyl sulphide (Me2S) is a stronger base than dimethyl oxide (Me2O) towards diborane due to hyperconjugation (Figure 6), whereas reverse is true for BF3 as acid.
In these cases. hyperconjugation involves the H+ ions in diborane for stabilizing the adducts as shown in Figures 5 and 6. Such inversions are more common in the later transition metal complexes.
1.6.2 STERIC EFFECTS
The steric affects are of no consequence for ionic reactions involving the small ion H+, but are significant in the Lewis acid–base reactions.
1.6.2.1 The F-Strain
Most important is the steric hindrance between the substituents on the central coordinating atom, It can be represented diagrammatically by sketching the structure of ethyldipropylamine and triethylborine adduct (Figure 1.6.3). This steric hinderance arises due to to the free rotation of the bigger alkyl groups in space. This phenomenon is known as the front strain or the F-strain.
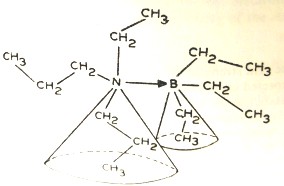
Figure 1.6.3 The F-strain due to the free rotation of
alkyl groups. Larger groups describe a larger-angled
cone for rotation around N – B single bond
This decreases the enthalpy of reaction between say, substituted pyridines and trimethylborane (BMe3). The F-strain is so much that 2-t–butylpyridine does not form a stable adduct with trimethylborane whereas pyridine forms a stable adduct (Figure 1.6.4). This can be seen from the enthalpy of reactions (ΔH) of these amines; more negative the ΔH is, greater is the stability of the adduct. (The stability of 2-methylpyridine adduct is, however, more than that of the triethylamine adduct due to inductive effects.)
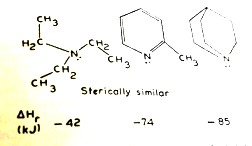
Figure 1.6.4 Enthalpy of reactions between pyridine,
2-methylpyridine (2-picoline) and 2-(t-trimethylpyridine
It is interesting to observe that stability of the trimethylborane adducts with triethylamine (–42) , 2-methylpyridine (–74) and quinolidine (without any F-strain) (–85) (ΔH in kJ mol–1) increases regularly with the basic nature of these amines.
1.6.2.2 The B-Strain
The B-strain or the back strain, is due to the steric requirements of the donor atom has been mentioned earlier (Figure 1.6.5).
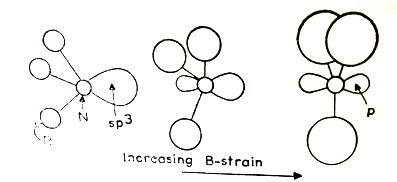
Figure 1.6.5 The B-strain as the alkyl group (R)
becomes bigger. The LP resides is tetrahedral
sp3 hybrid orbital for small R group, but
resides in pure p orbital with big R alkyl groups
For effective donation of the electron pair, it should be present in the strongly directional sp3 hybrid orbital. (In nitrogen atom, the sp3 hybrid orbitals have 25 % s and 75% p character. The bulky alkyl groups on N atom of amines can force the bond angles to open up. This increases the p character of the bonded orbitals and the s character of the orbital containing the lone pair of electrons. The effect is so much that tri-(t-trimethyl)butylamine becomes a planar molecule. The bonded atoms now reside in sp2 hybrid orbitals (with 33.3 % s and 66.7% p character) and the lone pair of electrons occupies sp hybrid orbital with 50% s and 50% p character); and the compound becomes neutral as the lone pair of electrons in sp hybrid orbitals is not at all suited for forming a coordinate bond and tri-(t-trimethyl)butylamine becomes a neutral compound Figure 4).
Similarly, for Lewis acid also, the strength of the acid depends on the nature of orbital used by the central atom for accepting the electron pair from the Lewis bases. In BR3, it may change from the sp3 to sp as the R group becomes bulky and may reduce the acid strength. It is observed that the acid strength of the Lewis acid also decreases with branching of the alkyl groups. For example, tri(t-trimethylbutyl)borane [B(CMe3)3] is so inert that it does not react with even a strong base methoxide (MeO–).
1.6.2.3 Internal Strain
Another type of strain, called the internal strain or the I-strain, is not well understood. It is observed in cyclic amines and ethers, where the base strength depends on the size of the ring also. Both B-strain as well as I-strain seems to operate in the behavior of these bases, but simple explanation of rules are available for the I-strain.
Therefore, no uniform scale exists for determining the strengths of Lewis acids and bases as the relative strength depends upon the reaction selected.